Galería de fotos









































Visitar enlace de:
Agencia Europea de Medio Ambiente
Asociación Española de Desalación y Reutiliazación (AEDYR)
Confederación Hidrográfica del Júcar
Fundación Ecología y Desarrollo
Consellería de Medio Ambiente, Agua, Urbanismo y Vivienda
Entitat de Sanejament d'Aigües (EPSAR)
Instituto Nacional de Meteorología
Ministerio de Medio Ambiente y Medio Rural y Marino
AJUNTAMENT DE BENISSA
|
|
SERVEI MUNICIPAL D´AIGUES POTABLES |
|
KEY DATA |
Updated the day 17/03/2014 |
| Municipality | BENISSA |
| Province | ALICANTE |
| Region | MARINA ALTA |
| Inhabitants | 13.932 |
| Inhabitants estimated in summer | 23.378 |
| Municipal area | 69,7 Km2 |
| Town hall | PLAZA DEL PORTA, 1 |
| CIF | A-0304100-A |
| Web | www.ayto-benissa.es |
| Management of the Service of Drinkable Water | MUNICIPAL, WITH MONOPOLY AND DIRECT MANAGEMENT |
| Offices of the Municipal Service of Waters | STREET COSTERA DEL POVIL, S/N |
| Telephone 24 hours | 901 41 01 02 Extension 3 |
| Fax | 965 73 28 40 |
| This email address is being protected from spambots. You need JavaScript enabled to view it. | |
| Web of the Water Service | www.aguasdebenissa.com |
| Year of beginning of the Water Service | 1.969 |
| Number of Sanitary Industrial Record | 27/00215/A |
| Number of users type I domesticate | 9.337 |
| Number of users industrial type | 122 |
| Monthly consumptions | see more ... |
| Municipal registered (two-monthly) consumption | see more ... |
| Grant Confederación Hidrográfica del Júcar | 2005CP0016 |
| Origin of the water | BENIGEMBLA, BENIDOLEIG Y BENISSA |
| Captation of the water | UNDERGROUND |
| Well San Antonio | see more ... |
| Well Manuel Torres | see more ... |
| Well Corralet | see more ... |
| Well Cami Sanet | see more ... |
| Well Canor | see more ... |
| Level of the wells | 50% |
| Capacity of storage | 20.000 m3 |
| Capacity tank Salvador Ivars - Collao | 2.500 m3 |
| Capacity tank Ibiza - Collao | 5.000 m3 |
| Capacity tank Europa - Collao | 10.000 m3 |
| Capacity tank regulating de Benimarco | 2.500 m3 |
| Treatment applied to the water | CLOI CHLORINATE GAS ó HIPOCLORITO |
| Analysis | see more ... |
| Water hardness | 17,07 ºF |
| Level of the tanks of the Collao | 307,20 METERS ON THE LEVEL OF THE MAR |
| Level of Benimarco's tank | 250,32 METERS ON THE LEVEL OF THE MAR |
| Functioning of the water network | FOR GRAVITY |
| Network of pipelines | 260 Km |
| Materials of the pipelines | FIBROCEMENTO, SMELTING, STEEL, POLYETHYLENE |
| Characteristics of the water network | BRANCHED OUT, PRINCIPALLY |
| Park of meters installed 5.685 |
WITH LESS THAN NINE YEARS OF ANTIQUITY 3.774 |
| Park of meters AQR (radio system) | 594 |
| Reading meter | TWO-MONTHLY |
| Invoice | TWO-MONTHLY |
| Water tariffs | B.O.P. 24-08-2005 |
| Tariffs of purification Generalitat Valenciana | D.O.G.V. 30-12-2013 |
| Investments year 2013 | 0 EUROS |
| Subsidies year 2013 | 1 |
| Record of breakdowns 2013 | 442 INTERVENTIONS |
| Processes for fraud year 2013 | 5 |
| Sanctions pecuniariarias for fraud year 2013 | 10.369,41 EUROS |
Water described as “hard” has a high concentration of dissolved minerals, specifically calcium and magnesium ions. Hard water does not present a health risk nor does it have a negative effect on the environment although it is a nuisance and causes added expenses.
Temporary hardness is caused by carbonates and can be eliminated by boiling or by adding lime -Ca(OH)2 (Calcium Hydroxide)- to the water. Calcium carbonate is less soluble in hot water than in cold so that when the water is boiled the bicarbonate is precipitated out of the solution leaving the water softer.
Permanent water hardness cannot be eliminated by boiling and is due to the presence of calcium and magnesium or chlorides, which are more soluble at higher temperatures. Despite its name this sort of hardness can be eliminated using sodium sulfate.
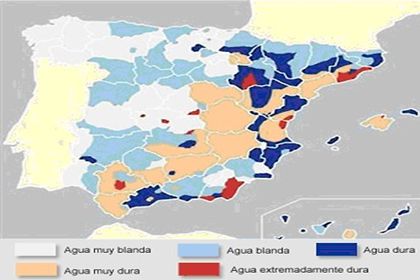
(Very soft water / Soft water / Hard water / Very hard water / Extremely hard water)
Map showing hard water zones in Spain
The drinking water supplied by the Municipal Water Service comes from the inland area of the Marina Alta, characterized by limestone mountains through which the rain water runs and filters to the aquifers resulting in concentrations of dissolved calcium of approximately 200 mg/l and therefore can be classified as HARD WATER.
The effects of hard water are:
- The use of more detergents for clothes washing. In laundering clothes larger quantities of detergents or soap have to be used in order to get good results. Hard water interferes with soap and detergent performance because the positively charged ions partially inactivate the principal active ingredients.
- More soap and shampoo for personal hygiene with poorer results because the same occurs as with detergent. It is more difficult to work up a lather resulting in dry skin and dull lank hair.
- More salt needed in the dishwasher to keep the appliance’s water softening systems clean permitting better results. If salt is not used dishes and glasses may be spotted and dull when dry. Lime-scale deposits can form on the water heating elements of the appliance contributing to inefficient operation or failure.
- Danger of blocked pipes. The accumulation of lime-scale, especially in hot water pipes and water heaters, can clog up pipes and reduce the life of water heaters.
Classification of the hardness |
|||||
| Mg/l de CaCo3 | º French | DegreesClark ºe | Deutsche Härte ºdH |
Water type |
|
| 0 - 3 | 3º | 1,19º | 0,95º | soft | |
| abr-60 | 3º - 6º | 1,19º - 4,20º | 0,95º - 3,35 |
moderately soft |
|
| 61 - 120 | 6º - 12º | 3,35º - 8,39º |
slightly hard |
||
| 121 - 180 | 12º - 18º | 8,39º - º10,05º |
moderately hard |
||
| 181 - 250 | 18º - 25º | 4,20º - 12,59º | 10,05º - 12,41º | hard | |
| . + 250 | .+ 25º | .+ 12,59º | .+ 12,41º |
Very hard |
|
Ways to prevent the negative effects of hard water:
- Laundry: According to the manufacturers, powder gives better results than liquid detergents in hard water. To obtain optimum results it must be taken into account that detergents are formulated for moderately hard water and therefore give dosage instructions according to the different sorts of water and degree of dirt. It is recommended to use higher doses in hard water areas and follow the manufacturers washing instructions. Unlike dishwashers, washing machines don’t have water-softening systems since 75% of the washing cycles used employ cold water and therefore no lime scale is produced. Water softeners can be used to neutralize the water’s hardness in hot washes.
When hard water is used for washing, lime scale can’t actually be seen on the clothes although these do become rough due to a build up of incrusted lime.
- Washing crockery & cutlery in a dishwasher: The first thing we should do is to adjust the setting of the appliance to the hardness of the water used. Dishwashers heat up the water used to over 35 to 40 degrees Celsius, temperature at which precipitation occurs and lime-scale is formed. In order to prevent this, these appliances use synthetic resin filters that trap the particles and soften the hard water. These filters must be washed regularly in order to maintain their efficiency. This is why salt is used.



Domestic appliances effected by hard water
- Skin and hair: Skin can be re-hydrated or lubricated if it becomes dry. For example after showering oil can be rubbed onto wet skin to get results 15 to 20 seconds. Another method is to apply body lotion although this is more expensive and time-consuming process. Hair is as delicate as the skin and can also effected by exogenous agents and should also be given special care in the form of shampoos with oily additives.
- Watering plants: Add a few drops of vinegar or lemon juice to the water,
- Cleaning taps: Wrap taps with toilet paper or bandages and soak these with vinegar. Leave overnight and then rinse with plenty of water.
- Cleaning the bathroom: Rub lime-scale with a cloth soaked in hot white vinegar. Rinse with plenty of water. The operation should be repeated until the stains have disappeared. If yellow stains appear these can be removed by rubbing with bleach.
- De-scaling iron soleplates: Fill the iron with vinegar and turn on until this evaporates. Then clean the vents in the soleplate with a cotton bud soaked in Vinegar.
The “gota fria” (literally translated: “cold raindrop”) is well known in the Marina Alta area. The last one hit the area on the 12th and 13th October 2007. Beniarbeig, Els Poblets and El Vergel were flooded when the Girona River burst its banks after 400 mm of rain. In Benissa numerous roadside walls collapsed and Calpe suffered floods when the normally dry Quisi overflowed.
This meteorological phenomena is typical in the Mediterranean and various episodes have been registered throughout the years along the Spanish Mediterranean coastal area.
The “gota fría” occurs when, at the end of the summer there is a contrast between the temperature of the sea and the cold polar air. The warm air rises rapidly to form a humid mass of low pressure or cyclone at high altitude that moves towards land and, on cooling down, causes the phenomena. In the Marina Alta the mountains create a barrier which is why the rain storms occur along the coast.
Apart from causing floods the “gota fría” usually generates strong winds of up to 150 k.p.h. provoking damages inland to trees and buildings. The phenomena also causes storms at sea with waves that damage beaches, promenades, and boats moored in the ports.

Gentle rain falls, those that sink in to the earth gradually and are welcomed by farmers, hardly ever occur in this area. Here the rainy seasons occur in autumn from September to November and in spring between February and May. The rain normally falls only for a few days but very heavily.
The rains that accompany the easterly strong winds are usually very heavy in the area where the sources of the Gorgos and Girona rivers, and the wells that supply water to Benissa, can be found. The porosity of the land means that the rain water quickly reaches the aquifers and after a “gota fria” the recuperation of water levels is spectacular. On the other hand the lack of rain can result in an important drop in the level of water in the aquifers, something that is known as a drought.
AFTER THE RAIN
After the rain:
Suns between the clouds and in the streams,
Sweets of almonds and of hazel-nuts,
Honeyed dates and hot bread.
After the rain:
My mother, my brothers and sisters
And our house of clay,
Our white doves.
After the rain:
Coloured rainbows of peace,
Without arms, without president.
After the rain,
... after the rain.
Muhsin Al Ramli